Heat of Neutralization
Purpose
The purpose of this experiment is to
determine the heat of reaction for a neutralization reaction.
![]()
Problem
to be Investigated
For which acid, HCL, H2SO4,
or CH3COOH, is the greatest quantity of heat released per mole of H+. Why?
Discussion
The literature indicates that 57.2 kJ
(13.7kcal) are released as heat for the reaction
![]()
However, a higher value may be obtained
in moderately concentrated solutions, and a lower value may be obtained if a
weak (partially ionized) acid is used.
In this experiment you will determine
the quantity of heat evolved when a strong base, sodium hydroxide, neutralizes
various acids.
Equipment
and Chemicals
Parr model 1451 solution calorimeter,
0.1 M HCL, 0.05M
H2SO4, 0.1M CH3COOH,
1.0 M NaOH (Know safety precautions!)
Directions
Carefully read the instructions
supplied by the instrument manufacturer.
(see attachment.)
The following modifications are
suggested:
1. Standardize
the instrument as directed.
2. Place 90 ml acid solution in the Dewar
flask. Place 10 ml 1.0 M base in the
rotating sample cell. This will give a
slight excess of base, assuring that all the acid is neutralized.
3. All liquid
solutions should be adjusted to nearly the same temperature before they are
loaded into the calorimeter.
4. Clean the
Dewar and sample cell thoroughly after each run.
5. In most
cases good results can be obtained with the recorder set on the 10 millivolt
scale (one degree full scale). If the pen
goes off scale during the run, change the temperature scale on the thermometer.
NOTE:
This experiment requires very careful work!
INSTRUCTIONS
FOR THE 1451 SOLUTION CALORIMETER
Introduction
The 1451 Solution Calorimeter described
in these instructions is an easily operated instrument which can be used to
measure the heat evolved or absorbed by chemical reactions in a liquid
media. Measurements are made at room
temperature and atmospheric pressure for systems producing energy changes
ranging from 2 to 1000 calories.
At the start of a test in this
calorimeter one liquid is held in a glass Dewar while the other reactant,
either solid or liquid, is held in a sealed glass rotating cell which is
immersed in the first liquid. After both
reactants come to thermal equilibrium, the operator starts the reaction by
depressing a push rod to drop the contents of the cell into the surrounding
liquid. The reaction then proceeds to
completion under the vigorous stirring action of the rotating cell. Throughout the test, temperatures in the calorimeter are sensed by
a thermistor and read from a bridge which is built into the calorimeter
case. Deviations from the bridge reading
are traced by a potentiometric strip chart recorder to produce a thermogram
showing the temperature change produced by the reaction. Data from the thermogram can then be used to
compute the change in enthalpy.
The instructions given in this manual
cover the basic steps to e followed in:
setting up and operating the calorimeter; recording temperature changes; standardizing the calorimeter and computing
energy values.
Calorimeter
Operations
Sample Size. The rotating sample cell will hold up to 20
ml of liquid sample or a solid sample weighing up to one gram. More than one gram of solid may be used in
some cases,s but smaller samples are preferred so that the heat capacity and
ionic strength of the system will not change significantly when the reactants
are mixed. The Dewar must be filled with
not less than 90 ml and not more than 120 ml of liquid to properly cover the
rotating cell.
Filling the Dewar. It is best to lift the Dewar out of the air
can during the filling operation. The
liquid to be placed in it can be measured volumetrically, or the Dewar can be
placed on a solution or trip balance and filled by weight. After filling the Dewar, set it in the air
can and gently push the spacer ring down as far as it will go.
Loading a Solid Sample. Solid samples should be suitable ground so
that they will dissolve quickly or mix uniformly with the liquid in the
Dewar. Place the 126C Teflon dish on an
analytical balance and weigh the sample directly into the dish. Be careful not to drop any of the sample into
the push rod socket. After the final
weighing, set the dish on a flat surface and carefully press the glass bell
over the dish to assemble the cell. Do
not grasp or press the thin-walled glass stem during this operation; it is fragile and will break easily. Instead, grasp the bell and press it firmly
onto the dish. Then lift the cover from
the calorimeter and attach the cell to the stirring shaft by sliding the
plastic coupling onto the shaft as far as it will go and turning the thumb
screw finger tight.
Hold the cover in a horizontal position and lower it carefully until the
bottom of the rotating cell rests on a firm, flat surface; then insert the push rod through the pulley
hub and press the end of the rod into the socket in the 126C sample dish.
Loading a Liquid Sample. Liquid samples can be measured into the
rotating cell either by volume or by weight.
Best precision is obtained by weighing,
but filling from a volumetric pipet may be adequate in some cases. Set the 126C Teflon dish on a flat surface
and press the glass bell over the dish, handling the glass carefully as
described above. If the sample is to be
weighed, tare the empty cell on a laboratory balance; insert a pipet through the glass stem and add
the liquid; then reweigh the cell. Attach the cell to the stirring shaft and
insert the push rod.
Install the thermistor probe in the
cover opening and press the bushing firmly into place to anchor the probe in
its proper position. Handle the cover
carefully after installing the probe since the glass stem will break easily.
Lower the cover assembly with the cell
and thermistor probe into the Dewar and set the cover in place on the air
can; then drop the drive belt over the
pulleys and start the motor.
Combining the Reactants. Each test in a solution calorimeter can be
divided into three distinct time periods:
(1) a preperiod during which the
reactants are allowed to come to thermal equilibrium; (2) a
reaction period during which the reactants are combined and an enthalpy change
occurs in the system; and (3) a postperiod during which the calorimeter
again comes to equilibrium. At the end
of the preperiod, start the reaction by pressing the push rod downward to drop
the sample out of the rotating cell.
This should be done quickly without interrupting the rotation of the rod
and without undue friction from the finger.
Push the rod down as far as it will go;
after which it should continue to rotate with the pulley. Let the stirrer continue to run during the
postperiod until a uniform slop is established, as explained later in these instructions.
Thermometer and Recorder Operations
The temperature measuring system is
this calorimeter consists of a thermistor probe and a special bridge designed
for use within the ten-degree span from 20o to 30o C. Within this range the response of the thermometer
is linear, with each 10 microvolt change in output representing a temperature
change of exactly .001o C. Thus, when
the output signal is fed to a recorder and plotted on a 1, 10, or 100 millivolt
chart, temperatures can be read directly from the chart without applying a
conversion factor. The following values
will apply when equating changes in thermometer output to temperature changes.
|
10 microvolts |
.000010 V |
= |
.001°C |
|
1 millivolt |
.001 V |
= |
.100°C |
|
10 millivolts |
.010 V |
= |
1.000°C |
|
100 millivolts |
.100 V |
= |
10.00°C |
Once these basic relationships are
understood the bridge can be balanced to a zero output at any baseline
temperature from 20° to 30° C and a
recording range can be selected to produce a full-scale trace corresponding to
a temperature change of 0.1°, 1.0° or 10°C. The true temperature at any point on the
chart can then be determined by adding the chart reading to the baseline
setting shown by the unit temperature switch and digital potentiometer located
within the marked box on the thermometer panel.
There are five switch positions on the
selector switch in the center of the thermometer panel, the middle three of
which have adjusting knobs.
OFF ZERO NULL
In the OFF position no power is
supplied to the thermometer and the system is dead. The switch should remain in this position
whenever the calorimeter is not in use.
The ZERO control adjusts the output of
the bridge to zero voltage.
The NULL control adjusts the bridge to
indicate a temperature of exactly 20°C at zero voltage output.
The
The READ position is used to feed the
thermometer signal to the recorder.
Calibrating the Recorder. Most strip chart recorders can be set to
produce a full scale trace with inputs of 1, 10, or 100 millivolts, which cover
the ranges generally used for the 1451 Calorimeter. The recorder should have a chart speed
selector and an adjustment for setting the pen to a zero baseline. Any specific instructions furnished by the
recorder manufacturer should be observed when using this equipment.
After connecting the recorder to the
thermometer bridge, balance the bridge and calibrate the recorder using the
procedure described below. These steps
should be taken in sequence when using the recorder for the first time. It is not necessary to repeat these
adjustments in each subsequent run, but the setting should be checked from time
to time to be sure that they have not changed.
1. Turn
the recorder on and turn the thermometer selector switch to ZERO for a warm up
period before making any adjustments.
Although the thermometer will usually warm up in ten minutes, a longer
period up to thirty minutes may be
required to reach maximum stability.
2. Start
the chart drive at a speed of one inch per minute.
3. Move
the recording pen to the zero baseline on the chart as instructed by the
recorder manufacturer.
4. Set
the range switch on the recorder to 100 millivolts (0.1 volt) full scale. In this position the full scale of the chart
represents a span of 10°C.
5. With
the thermometer selector switch in the ZERO position, use the ZERO adjustment
on the thermometer bridge to bring the pen back to its zero baseline.
6.
Turn the selector switch to NULL and bring the pen to its zero baseline
with the NULL adjustment.
7.
Turn the selector switch to
Example:
If the chart paper has 10 major units
in its ruling and the recorder is set at 100 millivolts (10.00°C) full
scale, each major unit on the chart represents 1°C. Therefore a reading of 4.52 units on the
chart indicates a temperature of 24.52°C in the calorimeter (20° baseline
+ 4.52° on the
chart scale).
Better precision can now be obtained by
changing the baseline setting and increasing the sensitivity of the recorder
after it is known that the temperature being measured is near 24.52°C. Move the temperature setting on the bridge to
exactly 24.000°C, then
change the range selector on the recorder to 10 millivolts (1.000°C) full
scale. If the pen then moves to 5.23
major divisions on the chart, the temperature in the calorimeter is 24.523°C (24° baseline
+ 0.523°C on the
chart).
Or, for best precision, set the
temperature dials to exactly 24.520°C and change the range selector on the
recorder to 1 millivolt (0.100°C) full scale. Now use the recorder as a null indicator and adjust
the digital potentiometer to bring the pen back to the zero baseline. If the digital meter then reads 0.525, the
temperature in the calorimeter is 24.525°C.
PRODUCING THE THERMOGRAM
Choosing the range. Before starting a run, try to estimate the total
energy change involved in the experiment so that the recorder can be set in a
range which will produce the best thermogram.
The following settings are recommended:
Up to 10 calories, set at .001 V (0.100°C) full
scale.
10 to 100 calories, set at .010 V
(1.000°C) full
scale.
100 to 1000 calories, set at .100 V
(10.00°C) full
scale.
If the sign and magnitude of the
enthalpy change cannot be estimated before starting an experiment, set the
recorder at .01 V (1.000°C)full
scale and make a trial run starting with a baseline in the middle of the
chart. The temperature change observed
in this experiment can then be used as a guide for setting the recorder to a
different range and baseline to produce a better thermogram in a subsequent
run.
Resetting the Pen. If the reaction to be examined is expected to
be endothermic, the pen must be raised to a higher position in order to record
a temperature drop during the reaction.
To raise the pen, simply turn the unit temperature switch or the digital
potentiometer to a lower setting. This
change can be made at any time without affecting the range and calibration of
the recorder.
In exothermic experiments, a baseline
adjustment may be desirable at this time if the temperature in the calorimeter
has changed significantly during the initial equilibration period.
Beginning the Trace. The liquid in the Dewar and the sample in the
rotating cell must reach thermal equilibrium, and the recorder must trace a
straight line for at least 3 or 4 minutes before starting the reaction. To minimize this equilibration period, the
reactants should be at approximately the same temperature when they are placed
in the calorimeter. This is particularly
important when working with reactions which produce low enthalpy changes. In such cases any temperature difference
between the two solutions in a liquid-liquid system should not exceed 0.2° when the
calorimeter is loaded. The calorimeter
should then be allowed to run for about 15 minutes before starting the
trace. Solid-liquid systems will usually
come to equilibrium within a shorter period.
Completing the Trace. Having established the initial drift, start
the reaction by depressing the push rod to open the rotating cell. This will produce a distinct temperature change
which will soon taper off to a uniform drift as the calorimeter again comes to
equilibrium. Continue the trace until
the drift line becomes straight and remains straight for at least three
minutes. Usually this condition will be
reached within ten minutes or less after starting the reaction.
At the conclusion of the test, stop the
recorder; lift the pen and turn the
thermometer selector switch to ZERO.
Remove the chart from the recorder and mark it to identify the run and
the reactants involved. Also, write in
the baseline temperature and show the recorder range setting for this run.
Emptying the Calorimeter. Stop the calorimeter motor; raise the cover carefully and wipe any excess
liquid from the parts that were immersed in the Dewar. Remove the thermistor probe from the cover
and remove the sample dish from the end of the push rod; then remove the rod and release the glass
cell from the drive shaft. Lift the
Dewar out of the air can and empty it;
then wash and dry all wetted parts carefully.
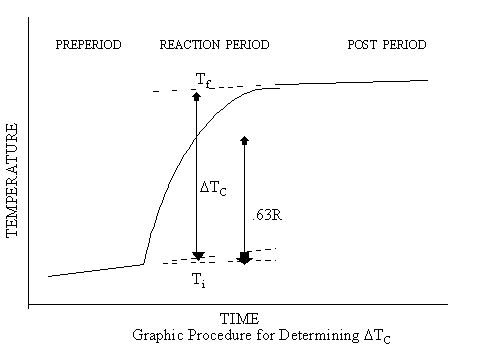
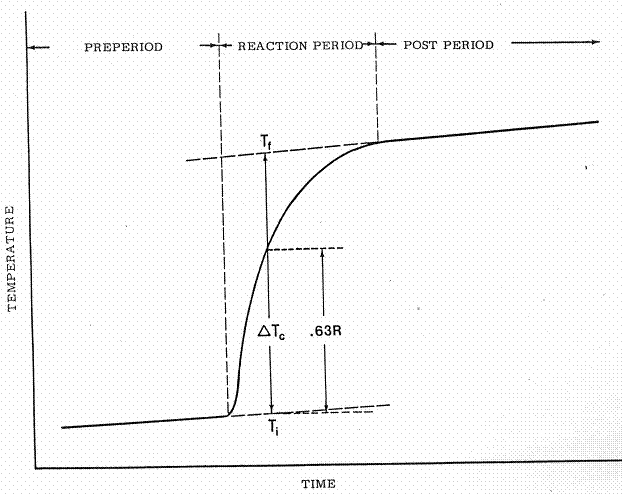
READING THE THERMOGRAM
In order to determine the net
temperature change produced by the reaction it is necessary to locate a point
on the thermogram at which the temperature reached 63 per cent of its total
rise (or fall). This can be done easily
using the graphic procedure which is described below, although other variations
of this method can be used as well.
1. Place a
straight edge over the preperiod drift line and extend this line well past the
point at which the cell was opened to start the reaction.
2. Move the
straight edge to the postperiod drift line and extrapolate this line backward
to the time when the cell was opened. If
there are fluctuations in the drift lines due to noise or other variations in
the signal, use the best average when drawing these extrapolations.
3. Using a
centimeter scale, measure the vertical distance, R, between the two
extrapolated lines at a point near the middle of the reaction period.
4. Multiply
the distance, R, by 0.63, then
5. Set the
zero end of the centimeter scale on the extrapolated preperiod drift line and
move the scale along this line to locate a vertical intercept with the
thermogram which is exactly 0.63R above the preperiod drift line. Draw a vertical line through this point to
intercept both draft lines.
6. Read the
initial temperature, ![]() ,and the final temperature,
,and the final temperature, ![]() , at the points of intersection with the drift lines and
subtract to determine the corrected temperature rise,
, at the points of intersection with the drift lines and
subtract to determine the corrected temperature rise, ![]() :
:
![]()
Although the thermogram shown
on page 14 to illustrate this graphic method is taken from an exothermic
reaction, the same steps can be used to established the corrected temperature
change for an endothermic reaction.
If it was necessary to reset the
pen during the test, the graphic procedure can still be used by taking into
account the two different baseline temperatures when reading the
intercepts. However, in such cases it
usually will be desirable to re-run the experiment using a different baseline
or a higher span on the recorder to produce an unbroken thermogram.
CALCULATING THE ENERGY CHANGE
The energy change, Q , measured in this calorimeter is
calculated by multiplying the net corrected temperature change, ![]() , by the energy equivalent, e, of the calorimeter and its
contents,
, by the energy equivalent, e, of the calorimeter and its
contents,
![]()
(Note that the energy equivalent, e, is
the same as the heat capacity, c.) If ![]() is measured in degrees
°C and e is expressed in calories per °C, Q
will be reported in calories.
is measured in degrees
°C and e is expressed in calories per °C, Q
will be reported in calories.
The energy equivalent, e , is determined by a calibration procedure
which is described below under Standardization.
The change in enthalpy, ![]() , at the mean reaction
temperature is equal to -Q divided by
the amount of sample used in the experiment, expressed either in moles or
grams.
, at the mean reaction
temperature is equal to -Q divided by
the amount of sample used in the experiment, expressed either in moles or
grams.
![]()
where T is the temperature.
Enthalpy values are usually
expressed in kilocalories per mole.
Procedures for converting enthalpy changes, ![]() , to thermodynamic
standard conditions and for using
, to thermodynamic
standard conditions and for using ![]() in other computations can be obtained from thermodynamics or
thermochemistry textbooks, or from literature references.
in other computations can be obtained from thermodynamics or
thermochemistry textbooks, or from literature references.
STANDARDIZING THE CALORIMETER
As explained above, in order to
calculate the energy change, Q ,
involved in a reaction, the energy equivalent, e, of the calorimeter and
its contents must be known. Values of e are determined by running several
calibration experiments in which the calorimeter is operated in the usual
manner but with reactants which release ( or absorb) a known amount of
energy. The energy equivalent in then
calculated by dividing the known energy input, ![]() , by the corrected
temperature rise,
, by the corrected
temperature rise, ![]() .
.
![]()
Standardization with TRIS. A sample of tris (hydroxymethyl)
aminomethane, commonly called TRIS, is furnished with the 1451 calorimeter to
provide a reliable standardizing reagent.
TRIS is furnished as a dry powder which can be used directly from the
bottle as supplied without further preparation, but undue exposure to air and
moisture should be avoided in order to
preserve the integrity of the standard.
For standardizing the 1451 calorimeter,
solid TRIS can be dissolved in dilute hydrochloric acid in a controlled
reaction for which the amount of heat evolved is well established. In the recommended standardization procedure
described below, 0.5 gram of TRIS is dissolved in 100 ml of 0.1 N HC1 to evolve
58.738 calories per gram of TRIS at 25°C.
This is the procedure:
1. Tare the Dewar on a solution or trip balance
and add exactly 100.00 ± 0.05
grams of 0.100 N HCl.
2. Weigh 0.50 ± 0.01 grams of TRIS into the
126C Teflon dish on an analytical balance to an accuracy of ± 0.0001 g.
3. Assemble the rotating cell; place it in the
calorimeter and start the motor.
4. Let the calorimeter come to equilibrium; the
set the recorder at .01 V
(1.000°C) full scale; set the
baseline at the bottom of the chart for an exothermic reaction and trace a
thermogram as previously described.
5. Analyze the thermogram to determine the net
corrected temperature
rise, ![]() .
.
6. Calculate
the known energy input by substituting in the equation:
![]()
where,
![]()
![]()
7. Calculate the energy equivalent of the
calorimeter and its contents by substituting in the equation:
![]()
where e will be expressed in calories per °C.
8. Determine the energy equivalent of the
empty calorimeter by subtracting the heat capacity of the 100 g of 0.1 N HC1 from e, as follows:
e’ =
e - (100.00) (.99894)
where, e’ =
the energy equivalent of the empty calorimeter in calories
per °C.
100.00
= the weight of 0.100 N HCl in grams
.99894
= the specific heat of 0.1 N HC1 at 25°C
Example:
A
standardization reaction involving:
0.5017
grams of TRIS, and
100.00
grams of 0.100 N HCl
|
|
produces
a net corrected temperature rise of:
![]()
In
the reaction the known energy input is:
![]()
The
energy equivalent, e, of the
calorimeter and its contents is then
computed:
![]()
The
energy equivalent, e’ , of the empty calorimeter in then computed:
![]()
EXAMPLE A - An Exothermic
Reaction
Problem: Determine the change in enthalpy for solid sodium sulfate,
![]() , when dissolved in a 5 gram/liter aqueous solution of barium
chloride,
, when dissolved in a 5 gram/liter aqueous solution of barium
chloride, ![]() .
.
![]()
![]()
![]()
![]()
Corr.
temp. rise ![]()
(from
chart, p. 20)
Energy
equivalent ![]()
Energy
evolved ![]()
Enthalpy
change ![]()
Or,
multiplying by 142.04 (the molecular weight of ![]() )
)
![]()
![]()
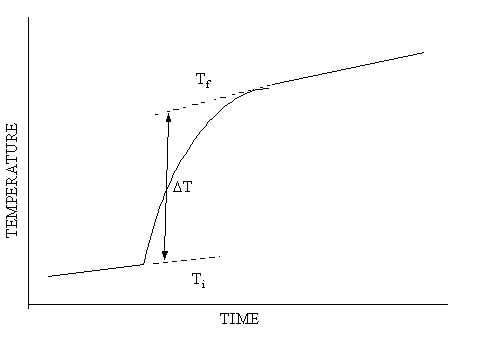
Example A:
An Exothermic Reaction
0.1458
g solid ![]() dissolved in 100.00 g of 5 g/l aqueous
dissolved in 100.00 g of 5 g/l aqueous ![]()
Full Scale
1mV = 0.1°C
Baseline = 24.852°C
Tf =
0.0494 + 24.852
Ti =
0.0074 + 24.852
DT = 0.0420°C
SAMPLE
CALCULATIONS
Experiment: Exp. 9.1 Heat of Neutralization
Data:
Standardization with TRIS
![]() , Dt=0.26°C
, Dt=0.26°C
|
Time (s) |
Volt (V) |
Temp (°C) |
Time (s) |
Volt (V) |
Temp (°C) |
|
0 |
-0.0219 |
17.81 |
360 |
-0.0195 |
18.05 |
|
60 |
-0.0205 |
17.95 |
420 |
-0.0194 |
18.06 |
|
120 |
-0.0202 |
17.98 |
480 |
-0.0194 |
18.06 |
|
180 |
-0.0199 |
18.01 |
540 |
-0.0193 |
18.07 |
|
240 |
-0.0198 |
18.02 |
600 |
-0.0193 |
18.07 |
|
300 |
-0.0196 |
18.04 |
|
|
|
![]()
![]()
HCl
|
Time (s) |
Voltage (V) |
Temp (°C) |
Time (s) |
Voltage (V) |
Temp (°C) |
|
0 |
-0.0115 |
18.85 |
120 |
-0.0019 |
19.81 |
|
15 |
-0.0026 |
19.74 |
150 |
-0.0019 |
19.81 |
|
30 |
-0.0017 |
19.83 |
180 |
-0.0020 |
19.81 |
|
45 |
-0.0018 |
19.82 |
210 |
-0.0020 |
19.80 |
|
60 |
-0.0018 |
19.82 |
240 |
-0.0020 |
19.80 |
|
75 |
-0.0018 |
19.82 |
270 |
-0.0020 |
19.80 |
|
90 |
|
|
300 |
-0.0021 |
19.79 |
|
105 |
-0.0019 |
19.82 |
|
|
|
Dt=0.98°C, ![]()
![]()
![]()
![]()
![]()
![]()
![]()
Calculations:
![]()
![]()
![]()
![]()
![]()