PARTICLE IN A BOX: VISIBLE SPECTRA OF DYES
Schroedinger
developed quantum mechanics based on the use of classical wave equations to
describe subatomic particles. One form
of the Schroedinger equation for a particle moving in one dimension is
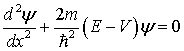
where
y = wave
function (No physical meaning, but y2 proportional to
probability of finding particle.)
m
= mass of particle
E
= total energy
V
= potential energy
![]()
The
“particle-in-a-box” is a description of a small particle moving in a box in
which the potential energy, V, is zero in the box, but is infinite outside the
box. The length of the box is “a”.
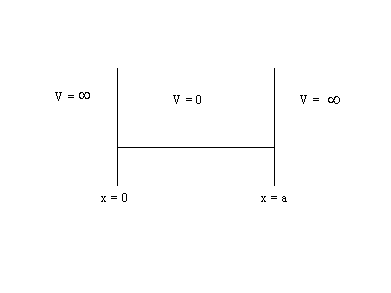
In
order to keep the particle in the box y must be zero outside the
box. Because y must be continuous, y must also
be zero at ![]() and
and ![]() .
.
One
solution to this problem is
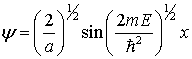
At ![]() ,
, ![]() ; thus
; thus
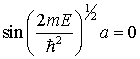
But also
![]()
Where ![]()
So,
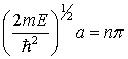
or
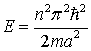
or
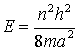
(n
is a quantum number 1, 2, 3, ...)
According
to our results, the energy levels are quantized!
The
Pauli exclusion principle requires that there be no more than two electrons in
any energy level. For molecules with N p electrons
there will be N/2 levels. If an electron jumps from the level n = N/2 to the lowest empty level, N/2 + 1, the change in energy, ![]() , is
, is
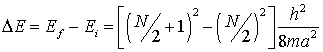
Since ![]()
Where ![]()
n = frequency
c = speed of light
l = wavelength
![]()
![]()
![]()
or
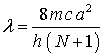
For
the series of compounds we will study
![]()
where j is the number of double bonds in the polyene chain between two
rings and ![]() the C---C bond length
of order 1.5.
the C---C bond length
of order 1.5.
Also
N = 2j + 4. In addition the
highly polarizable benzene rings require that we increase the length of the box
by L.
So that
![]()
or
![]()
Then
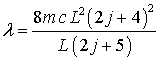
By
increasing the box length by L
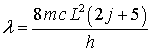
The
above equation may be used to estimate the wavelength of light absorbed by an
electron of mass, m, in a polyene
chain.
PURPOSE
The
purpose of the experiment will be to test the particle-in-a-box model. For an
electron in a box the wavelength of maximum absorption is given by
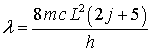
EQUIPMENT
AND CHEMICALS
Spectrophotometer
(Turner 350, Coleman 124, P. E. Lambda 3)
Methanol
Cyanine
dyes (![]() stock solutions in methanol, referred to as
stock solutions in methanol, referred to as
#I,
#II, #III.)
Compound (I)

1,1’-DIETHYL-2,2’-CYANINE
IODIDE
Compound (II)
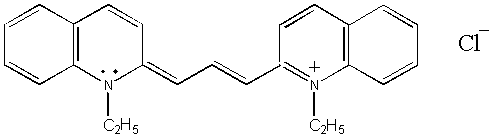
1,1’-DIETHYL-2,2’-CARBOCYANINE
CHLORIDE
Compound (III)
(III)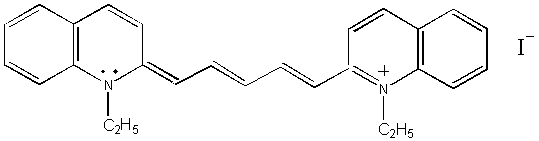
1,1’-DIETHYL-2,2’-DICARBOCYANINE
IODIDE
#II
is also known as pinacyanol chloride.
PROCEDURE
From
![]() stock solution,
prepare the following solutions (Use 100 microliter micropipet.)
stock solution,
prepare the following solutions (Use 100 microliter micropipet.)
#I Dilute 0.10 ml to 10 ml in methanol
Dilute
0.10 ml to 25 ml in methanol
#II Dilute 0.10 ml to 25 ml in methanol
Dilute
0.10 ml to 50 ml in methanol
#III Dilute 0.10 ml to 25 ml in methanol
Dilute
0.10 ml to 50 ml in methanol
For
each dye you will have two solutions of different concentrations. Scan each solutions as follows:
#I 470 - 550 nm
#II 575 - 635 nm
#III 675 - 735 nm
Use the graph showing absorbance (A) vs. wave length (l) for each solution.
Use
Beer’s law to determine the molar absorptivity for each solution of each dye.
![]()
A = Absorbance
a = molar absorptivity
b = path length in cm
c = concentration, mol/L
For each solution calculate a.
For a given dye the two values of a
should agree within five per cent.
For
each dye calculate maximum l for the
electron-in-a-box and compare it with your experimental values.
Literature
values for maximum l are the
following:
Dye
#I 525 nm
Dye
#II 610 nm
Dye
#III 705 nm
(From Introduction to Quantum Concepts in Spectroscopy, W. G. Laidlow,
1970, p. 35.)