CRYSTAL VIOLET REACTION
Purpose
The purpose of this experiment is to
determine how the rate constant for a reaction changes with ionic strength of
the solution and with temperature.
INTRODUCTION:
The experiment follows a procedure described in J. Chem. Ed., 41, 48 (1964). By using the UV-VIS spectrophotometer the
rate constant k is measured for the reaction
![]()
Here
![]() is crystal violet, a violet-colored
organic dye of molecular weight 408.0, which reacts with
is crystal violet, a violet-colored
organic dye of molecular weight 408.0, which reacts with
THEORY:
We assume the rate law for reaction (1) is
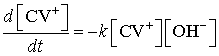
If ![]() , then
, then ![]() remains approximately constant during
the reaction, and
remains approximately constant during
the reaction, and
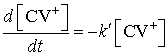
where ![]()
Integration
of (3) between ![]() and
and ![]() yields
yields
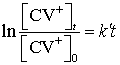
If
concentrations are determined by a spectrophotometer we can use the relation
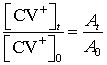
where A
is the absorbance.
Using
(5) and
(6) we obtain
![]()
Thus
![]() can be determined from
a plot of ln
can be determined from
a plot of ln ![]() vs. t; because (
vs. t; because (
According to transition-state theory
the rate constant at a given temperature for a bimolecular reaction varies with
the total ionic strength, m, of the system,
![]()
mcan be calculated from the charges, ![]() , and the molarities,
, and the molarities, ![]() , of all ions in the system:
, of all ions in the system:
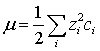
The
value for B can be determined (if the plot of ![]() vs.
vs. ![]() is linear) and
compared with the theoretical value, thereby testing the validity of the theory
for this system. (B is the slope.)
is linear) and
compared with the theoretical value, thereby testing the validity of the theory
for this system. (B is the slope.)
The
Arrhenius activation energy, ![]() , appears in the equation
, appears in the equation
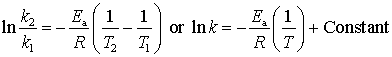
From
a plot of ![]() vs.
vs. ![]() (absolute
temperature), at constant ionic strength,
(absolute
temperature), at constant ionic strength, ![]() can be
determined if the plot is linear. The
slope is
can be
determined if the plot is linear. The
slope is ![]() .
.
EQUIPMENT AND CHEMICALS
Visible spectrophotometer (P. E. Lamba 3, Turner 350, Coleman 124)
Crystal violet (0.03 g/l),
Electrolyte solutions may be
prepared by dissolving a quantity of KNO3 in
0.008M NaOH (For example 0.002 mole KNO3
dissolved in 50 ml 0.008M
NaOH.)
PROCEDURE:
A stock solution containing 0.030g
of CV+.Cl- per liter is
available. Part A of the experiment
requires the determination of k at
three different ionic strengths but at one temperature, 25°C. For each determination, 50 ml. of 0.006g./l solution of CV+Cl- is
mixed briefly with 50 ml. of an
electrolyte solution; a portion of the mixture is then placed in the sample
cell ( water is in the other cell), and values for
absorbance of solution are recorded every minute for about 15 minutes.The spectrophotometer wavelength is set at the position
of highest absorbance (about 586 nm)
as determined with a non reacting solution of CV+Cl-. The first 50 ml. portion of electrolyte used is 0.008 M in NaOH; the portion used for the
second run is 0.008 M in NaOH and 0.04 M
in KNO3; for the third run the concentrations in the 50ml. portion are 0.008 M NaOH and
0.16 M KNO3. (Note: You cannot get a solution that is
0.008M NaOH
and 0.04M KNO3 by mixing
0.008M NaOH
and 0.04M KNO3. Since the
solutions are diluted 1:1 in each other, concentrations are reduced by
one-half.)
In part B of the experiment, k is
determined at the temperatures 25°C, 30°C, and 35°C, with the 50ml. electrolyte portion being 0.008 M in NaOH and no KNO3
present. As before the other 50 ml. portion to be mixed contains 0.006g. of CV+Cl-/l.
CALCULATIONS:
For each run calculate ![]() and
and ![]() at every time t.
Plot
at every time t.
Plot ![]() vs. t and determine
vs. t and determine ![]() ; then calculate k
from equation 4. For runs in part A calculate and determine
B in equation 9. Remember, the 50 ml. electrolyte portions were diluted
1:1. From the runs in part B determine
; then calculate k
from equation 4. For runs in part A calculate and determine
B in equation 9. Remember, the 50 ml. electrolyte portions were diluted
1:1. From the runs in part B determine ![]() in (11). Prepare tables and graphs displaying the
above data and results. Discuss the
validity of equations 8 and 11 for this system.
in (11). Prepare tables and graphs displaying the
above data and results. Discuss the
validity of equations 8 and 11 for this system.