ELECTRICAL CONDUCTANCE
PURPOSE
The purpose is to determine the
conductances of solutions of a salt, strong acid, weak acid, and slightly
soluble electrolyte.
From the data the ionization
constant of the weak acid and solubility product constant of the slightly
soluble salt may be calculated.
DISCUSSION
Conductance is defined as the
reciprocal of resistance.
![]()
It
is expressed as “reciprocal ohms” or “mho” (“ohm” spelled backward). “Specific conductance” is ohm-1 cm
-1
Specific conductance decreases as
the concentration of ions decreases.
“Equivalent
conductance”, L, is defined as
![]()
where
C is the concentration.
The equivalent conductance in a
solution in which the ions are far enough apart not to interact (infinite
dilution) is known as ![]() , equivalent conductance at infinite dilution. The ions act independently, and
, equivalent conductance at infinite dilution. The ions act independently, and ![]() is the sum of the
limiting conductances of each ion.
is the sum of the
limiting conductances of each ion.
![]()
(Values
of single ion conductances may be found in the CRC Handbook of Chemistry and
Physics.
![]() may be determined by
plotting L vs
may be determined by
plotting L vs ![]() and extrapolating to zero concentration. However, this is not successful for a weak
electrolyte because the degree of ionization increases with dilution and the
and extrapolating to zero concentration. However, this is not successful for a weak
electrolyte because the degree of ionization increases with dilution and the ![]() curve is not linear.
curve is not linear.
![]() for acetic acid may be
determined from
for acetic acid may be
determined from ![]() of the ions.
of the ions.
![]()
For a weak electrolyte the degree of
dissociation is

Consider
a weak acid:
![]()
Concentrations:
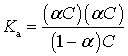
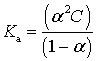
For the slightly soluble salt
![]()
However,
since the conductance, L, is very
small, the conductance of pure water should be subtracted. Thus
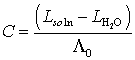
The
solubility product constant can then be calculated from the concentration of
the salt.
EQUIPMENT AND CHEMICALS
A.C. conductance bridge (YSI or
Beckman), conductivity cells, 0.100 M
NaCl, 0.100 M HCL, 1.0 M CH3COOH, saturated solution
of PbSO4. (Solution
concentrations need not be exactly 0.1000 M,
but should be known to three significant figures.)
DIRECTIONS
1.
Read the
instructions for using conductance bridges supplied by the manufacturer. Both instruments are essentially the
same. You have a choice of two A.C.
frequencies. Multipliers and scale dials
are adjusted to give a minimum on the null meter (Beckman) or wide shadow
(YSI). At that point the conductivity is
equal to
![]()
2.
Extreme
care must be made in making solutions and successive dilutions. The strong electrolytes are diluted by
one-half so that you have these solutions:
0.025, 0.0125, 0.00625 M,
0.00313 M. Acetic acid is diluted
similarly.
3.
Measure
conductance of distilled water first.
Then measure solution conductances, starting with most dilute
solution. After each reading wash the
cell with portions of the next solution.
4.
Wash some
solid PbSO4 with successive portions of distilled water to remove
any soluble impurities. Then determine
the specific conductance of a saturated PbSO4 solution. For PbSO4,![]()
5.
Ordinary
distilled water is not satisfactory since it has too high a conductance, mostly
due to dissolved CO2. Much
better water can be obtained by boiling distilled water to free CO2 and
capping a full bottle while it is hot,
It’s specific conductance should be ![]() or less. (200,00 ohm
resistance).
or less. (200,00 ohm
resistance).
6.
Since
conductivity is temperature dependent, the experiment may be run in a constant
temperature water bath.
UTILIZATION OF DATA
1.
For each
series of solution graph L
vs ![]() . If a straight line
is obtained use a least squares program on the computer to determine
. If a straight line
is obtained use a least squares program on the computer to determine ![]() (the intercept). Compare results for strong and weak
electrolytes. If the points are
scattered make more measurements to define a smooth curve. Compare
(the intercept). Compare results for strong and weak
electrolytes. If the points are
scattered make more measurements to define a smooth curve. Compare ![]() for each strong
electrolyte with accepted values.
for each strong
electrolyte with accepted values.
2.
For CH3COOH,
calculate Ka.
3.
For PbSO4
calculate Ksp.
SAMPLE
CALCULATIONS
Experiment: Exp. 9.4 Electrical Conductance
Data:
|
conc (mol / L) |
L ( |
|
0.05 |
111.8 |
|
0.025 |
105.6 |
|
0.0125 |
104.0 |
|
0.00625 |
110.4 |
|
0.003125 |
121.4 |
Calculations:

 )
)