Vibrational-Rotational Spectra of Gases
Purpose:
The purpose of this experiment is to:
- determine the infrared spectrum of a diatomic gas;
- to calculate vibrational force constants, vibrational energies, and the moments of inertia; and,
- to determine the effect of changes in isotopic mass upon the fundamental vibrational frequency, the vibrational force constant, and the moment of inertia of a diatomic gas molecule.
Equipment and Chemicals:
- A Fourier Transform-Infrared Spectrophotometer equipped with a gas sample cell.
- Cylinders of dry HCl and HBr.
Directions:
See the instructor for operating instructions for the FT-IR.
Note:
- Fill the cell in a hood.
- Dry the gases before filling the cell.
Calculations:
By examining the spectra, one can determine the value of the fundamental vibrations of HCl and HBr and of any overtones present. The fundamental vibration is
w, in units of wave numbers, .From this data, one can calculate the force constant for the fundamental vibration by using the relationship:
![]()
where, k = the force constant,
m = reduced mass, w = wave number,c = speed of light,
m = mass of the atom.
Determine the wave numbers or wavelengths at the peaks corresponding to changes in rotational quantum number.
The difference, in wave numbers, between adjacent lines (except at the origin) in the rotation-vibration spectrum is equal to 2B.
![]()
where, the moment-of-inertia, I, is given by
![]()
and r is the internuclear distance, and, .
m = the reduced mass.Calculate I, the moment of inertia, for HCl and HBr and the interatomic distances.
Determine the fundamental vibrational frequency of HCl and DCl.
Compare the ratio of the experimental determined frequencies with the theoretical relationship
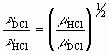
where,
n = vibrational frequency,and,
m = the reduced mass.For each gas, calculate the force constant for the fundamental vibration, from the relationship
![]() .
.
Calculate the moment-of-inertia and the internuclear distance for both HCl and DCl.