COLLIGATIVE PROPERTIES
Purpose
The purpose of this experiment is to investigate
colligative properties of solutions and how they can be used to determine
molecular weight of solute.
Discussion
From the Clausius-Clapeyron equation,
(1)
the vapor pressure of a solution of a non-volatile
solute may be compared to that of the pure solvent. It can be shown that
the boiling point elevation is
(2)
where T0 is the boiling point of the solvent,
M1 is molecular weight of solvent, DHvap
is
heat of vaporization of solvent, and m is the molal concentration.
This equation is valid for ideal solutions and for small temperature changes.
The equation may be rewritten as
(3)
where m is the molal boiling point constant. Since the molal concentration is
where,
W2 = mass of solute in grams,
M2 = molecular weight of the solute,
W1 = mass of solvent in grams.
Combining both equations the molecular weight
of the solute is
Analogous equations may be derived for freezing
point depression.
(6)
(7)
Values of Kb and Kf are best
determined experimentally, but may be determined from equation 2, as presented
in the following table:
| Solvent | B.P.
(oC) |
Kb
(oC / molal) |
M.P.
(oC) |
Kf
(oC / molal) |
| Water | 100.0 | 0.52 | 0.0 | 1.86 |
| Benzene | 80.2 | 2.53 | 5.50 | 5.12 |
| Carbon Tetrachloride | 76.6 | 5.03 | ||
| Camphor | 178.0 | 37.7 | ||
| Cyclohexane | 6.6 | 20.4 |
Equipment and Chemicals
Boiling Point:
Cottrell boiling point apparatus, Beckman thermometer, micro-burner, solvent (CCl4), naphthalene, other solute ("unknown", diphenyl, benzoic acid, salicylic acid, p-nitrotoluene).
Melting Point:
Beckman freezing point apparatus, Beckman thermometer, cyclohexane, naphthalene, other solute ("unknown", such as p-dichlorobenzene, biphenyl, p- bromochlorobenzene).
Directions
Boiling Point:
- Pipet 75 ml CCl4, with a bulb, into the apparatus.
- Insert Beckman thermometer. Note: this is a special thermometer with a six degree range. Each division is 0.01o. The mercury in the reservoir may be varied to coincide with the desired temperature. Thus, absolute temperatures are not measured, only temperature changes. A hand lens may be used to facilitate reading the thermometer.
- Boil solvent gently and determine boiling point. The thermometer bulb must be in contact with the boiling solution, not the condensing vapor which is pure solvent. Take several readings, which should agree with 0.003o.
- Add an amount of naphthalene which will give a 1-2o temperature change. Measure the boiling point as before. (Use pellet press to facilitate sample handling.)
- Add another quantity of naphthalene and again determine the boiling point.
- From the data determine two values of Kb and the average Kb. (Use a rearranged equation 5.)
- Pour solution into waste solvent bottle, not the drain.
- Repeat steps 1 - 5 with an "unknown" instead of naphthalene.
- Determine average molecular weight of "unknown". Use equation 5.
Directions
Melting Point
- Pipet 20 ml (to nearest 0.01 ml) cyclohexane into apparatus.
- Place tube in larger test tube (air jacket) which is submerged in ice bath. Freezing point of cyclohexane is measured with Beckmann thermometer to nearest 0.002o on a cooling cycle. Melt and repeat.
- Dissolve about 0.03 g (measured to 0.0001 g) naphthalene. To determine melting point, the solution is frozen to a slurry, the test tube is placed in the air jacket, and the solution is allowed to warm to several degrees above the melting point. Record the temperature at 30 second intervals. Plot temperature vs. time. The melting point is the intersection of the two straight-line portions of the curve. Repeat until melting points differ by no more than 5%.
- Add another quantity of naphthalene and again determine melting point.
- From the data determine Kf.
- Pour solution into waste solvent bottle, not the sink.
- Repeat steps 1 - 4 with an "unknown" instead of naphthalene.
- Determine molecular weight of "unknown".
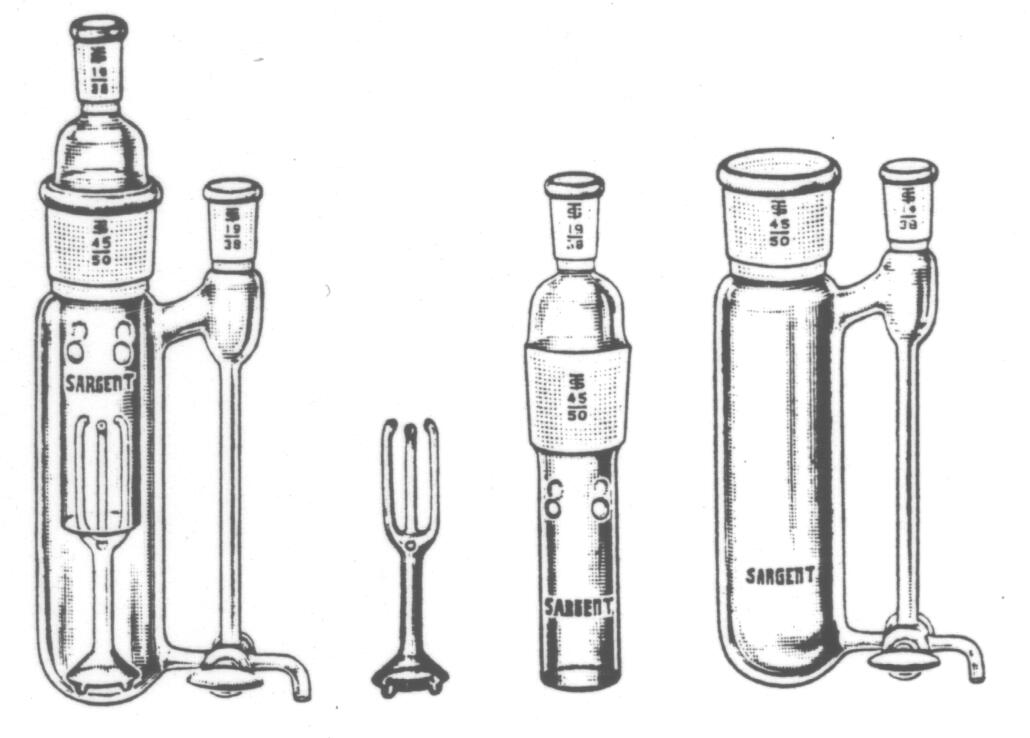
Molecular Weight Apparatus-Boiling Point,
Cottrell-Choppin, T. Grindings, PYREX Brand Glass.
Basic Cottrell design, but of heavy construction
with separate pump, reducing fragility and a three way stopcock permitting
the withdrawal of samples from boiling tube or condenser or the return
of condensate to the vessel. For technique, refer to Journal of Chemical
Education, 24, 491 (1947). For thermometer with T grinding,
see S-62004. For condenser, see S-22530-B. T grindings, outer part, No.
19/38 are provided to receive thermometer and condenser. The inner and
outer elements are joined by a T grinding No. 45/50. Overall height, 310
mm: overall width, 115 mm.
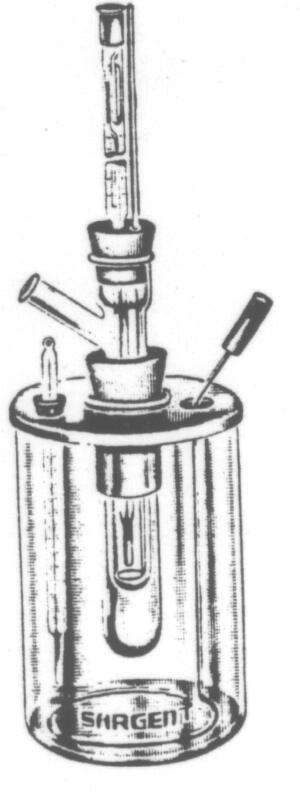 Molecular
Weight Apparatus- Freezing Point, Beckmann
Molecular
Weight Apparatus- Freezing Point, Beckmann
Improved form, intended for use of sample pellets
prepared in presses. Consists of a freezing point tube, 190 mm by
25 mm, with a side tubulature for the introduction of pellets, tub
being supported by a rubber stopper in an air jacket tube 150 x 37 mm
which rests in a corresponding hole in a nickel plated brass cover fitted
to a glass freezing job, S-43725-D.
The freezing point tube is equipped with stopper
through which are inserted a Beckmann thermometer and a chromel wire stirrer,
the latter operating through a small piece of glass tubing inserted in
the stopper. The internal stirrer is finished in a ring form agitating
end which moves vertically within the freezing point tube and is so adjusted
as to clear the Beckmann thermometer bulb when located in the center of
the tube.
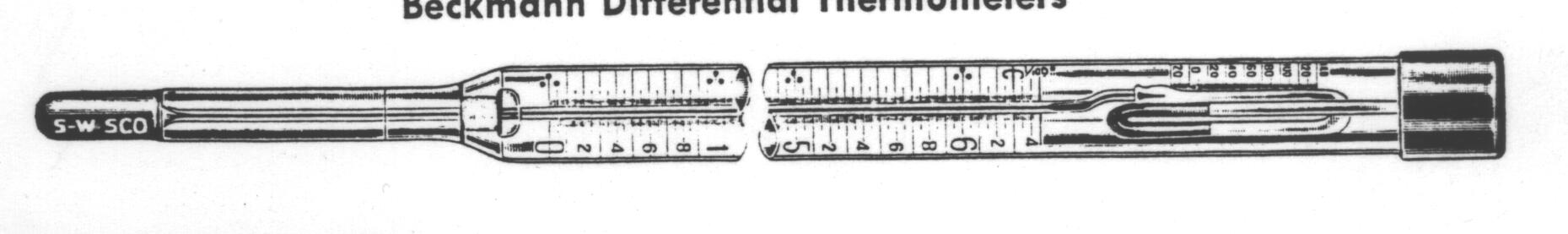
Thermometer-Beckmann Differential, Upward
Scale
For accurate measurements of very small temperature
differentials such as those encountered in boiling point determinations
and calorimeter measurements. The range covers approximately 5oC
graduated in 1/100o subdivisions. Has a mercury reservoir with
auxiliary scale providing a rough indication of the amount of mercury to
be trapped in the reservoir to provide the desired operating range. A dropping
trap is included. This instrument can be used only for determining temperature
differences.
Thermometer-Beckmann Differential, Downward Scale
Similar to the 14-5905 Thermometer, but for measurement
of small differentials in freezing point and molecular weight determinations,
and with scale reading downward. Subdivided to 1/100o.