Liquid-Vapor Equilibrium
Purpose
The purposes of this experiment are to
measure the vapor pressure of a pure liquid as a function of temperature and to
use that relationship to calculate the heat of vaporization.
Discussion
For any two phases in equilibrium, the
free energies must be equal
![]() (8-1)
(8-1)
A small change in free energy in one
phase must be balanced by a corresponding change in the other phase.
![]() (8-2)
(8-2)
but ![]() ; so
; so
![]() (8-3)
(8-3)
This may be rearranged to give
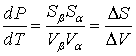 (8-4)
(8-4)
According to the Second Law of
Thermodynamics
![]() (8-5)
(8-5)
Substituting into equation 8-4,
![]() (8-6)
(8-6)
Now let us consider the liquid-vapor
equilibrium. Since the volume of a gas
is much larger than that of the same mass of liquid, we may assume that ![]() . Let us also assume
that the vapor behaves as an ideal gas;
that is,
. Let us also assume
that the vapor behaves as an ideal gas;
that is, ![]() . Thus equation 8-6
becomes
. Thus equation 8-6
becomes
![]() (8-7)
(8-7)
![]() is now the enthalpy of
vaporization. From calculus, we know
that
is now the enthalpy of
vaporization. From calculus, we know
that
![]() (8-8)
(8-8)
and
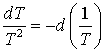 (8-9)
(8-9)
So, we can rewrite equation 8-7,
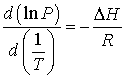 (8-10)
(8-10)
When we plot ![]() vs.
vs. ![]() ,
, ![]() is the slope of the
curve. If we plot
is the slope of the
curve. If we plot ![]() versus
versus ![]() , the slope will be
, the slope will be ![]() .
.
Thus the heat of vaporization can be
calculated from Pressure-Temperature
data.
In this experiment you will measure the
boiling point at various pressures.
Equipment
and Chemicals
Cottrell-Ramsay-Young boiling point
apparatus, mercury, manometer, ballast bottle, and aspirator or cacuum pupm,
microburner, barometer, hexane, cyclohexane, ethanol, or other volatile liquid.
Procedure
The apparatus should be assembled as
shown in Figure 8-1. Care should be
taken to see that the system is air-tight.
Place some liquid in the boiling
apparatus. Boiling chips may be used to
prevent bumping. Evacuate the system to
the desired pressure. Carefully heat the
liquid to boiling. Avoid superheating
and see that the boiling liquid percolates over the thermometer bulb. Determine the boiling pint at that given
pressure. The pressure inside the system
is the atmospheric pressure minus the difference in height of the two mercury
columns in the manometer.
Add air to increase the pressure
approximately four cm. Again determine
the boiling point. Repeat at other
pressures to get a wide range of pressures and temperatures.
Repeat with other samples of the same
liquid.
CALCULATIONS
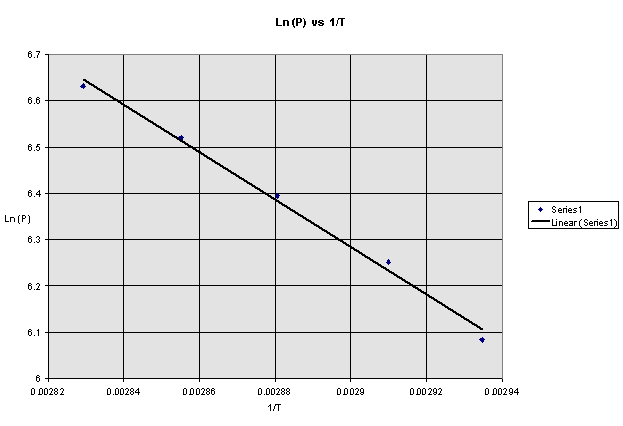
Plot a graph of ![]() versus
versus ![]() . Determine the slope
and use equation 8-10 to calculate the heat of vaporization. Also, use computer programs (LNREG1) to find
slope.
. Determine the slope
and use equation 8-10 to calculate the heat of vaporization. Also, use computer programs (LNREG1) to find
slope.
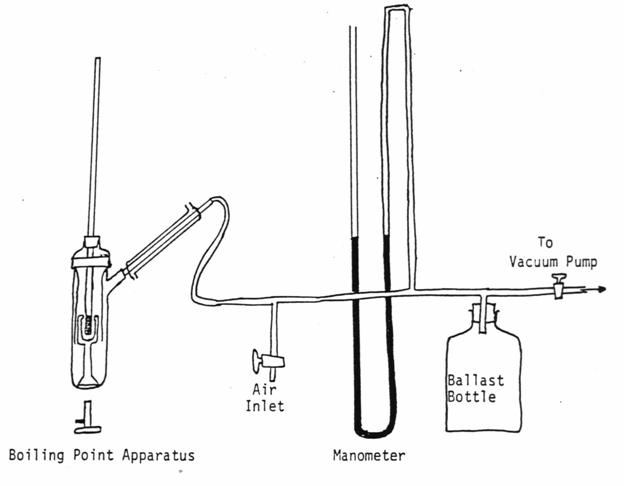
Figure 8-1. Cotttrell,Ramsay-Young Vapor Pressure
Apparatus
SAMPLE
CALCULATIONS
Experiment: Enthalpy of Vaporization
Sample
Data:
|
Pressure (mmHg) |
Boiling Pt. (°C) |
Boiling Pt. (K) |
ln P |
1/T (x 10-3 K-1) |
|
754.4 |
80.1 |
353.1 |
6.626 |
2.832 |
|
714.4 |
78.2 |
351.2 |
6.571 |
2.847 |
|
674.4 |
76.5 |
349.5 |
6.514 |
2.861 |
|
634.4 |
74.45 |
347.45 |
6.453 |
2.878 |
|
594.1 |
72.5 |
345.5 |
6.387 |
2.894 |
Calculations: Performing a linear regression gives the
slope and y-intercept for the line of best fit through the data. For the above data, the slope is -3844.75
K. Thus, the value for the Heat of
Vaporization (DHvap) of
cyclohexane is determined to be:
DHvap = -
(slope) x R = -(-3844.75 K) x 8.314 J/K mol
=-31965.25 J/mol