Heat of Combustion
PURPOSE
The purposes of this experiment are to determine
the heats of combustion of several related substances using a bomb calorimeter
and relate differences in heats of combustion to structural differences.
DISCUSSION
A detailed procedure for operating a bomb calorimeter
is found in the manual accompanying the instrument. A cross section of
a plain calorimeter is shown in Figure 6-1. The essential features are
thermometer (A), water bucket (B), and combustion bomb (C), also shown
in Figure 6-2. The bomb contains the sample, oxygen, and fuse wire to ignite
the sample.
As a sample is burned, the heat produced increases
the temperature of the water in the bucket. The temperature rise is indicated
by a thermometer in the water, which is stirred to insure even distribution
of heat. A sample of known heat of combustion is burned to determine the
heat capacity of the system. This then is used to determine the heat of
combustion of the unknown sample.
Since the bomb is of constant volume, not constant pressure, the heat of combustion calculated is DE (or DU), not DH. However, DH can be calculated provided the chemical equation is known.
![]() (6-1)
(6-1)
![]() (6-2)
(6-2)
Where Dn is the change in the number of moles of gas (the number of moles of gas products minus the number of moles of gas reactants in the balanced chemical equation)
EQUIPMENT AND CHEMICALS
Parr oxygen bomb calorimeter (or equivalent),
pellet press, thermometer (0.01oC), fuse wire.
Oxygen, benzoic acid (combustion standard), sucrose,
glucose, ascorbic acid, acetylsalicylic acid, naphthalene, or other combustible
organic solid.
PROCEDURE
Carefully read the instruction manual for the
calorimeter.
The following general operating instructions
should be observed:
- Form and weigh a pellet (not to exceed 1.1g) of sample and place it in cup.
- Attach 10 cm fuse wire to electrodes with wire touching top of pellets (See Figure 6-2) and place in bomb.
- Fill the bomb with oxygen to pressure of 25 atmospheres. Release pressure and again fill with oxygen. This removes most of the nitrogen and reduces the necessity for correcting the nitric acid formed.
- Place the bomb in the bucket containing two liters (volumetric flask) water at a temperature two or three degrees below room temperature. Check to see that electrical wiring is correct and that there is no short circuit.
- Press firing button to ignite sample.
- Determine the change in temperature.
- Remove the bomb, release the pressure, open the bomb, and remove and measure the length of the remaining fuse wire.
The following procedure should be observed when
determining heat of combustion.
Determine the heat capacity of the system by
igniting a pellet of benzoic acid. Take temperature readings for several
minutes before ignition and after ignition until the temperature begins
to decrease slightly. Plot a graph of temperature vs. time and extrapolate
to ignition time in order to determine the temperature change.
Release the pressure and dry the bomb. There
should be no carbon deposits inside the bomb. If there are, repeat with
a smaller sample. Repeat the process using a pellet formed from some of
the dry sample. (Figure 6-3)
CALCULATIONS
The heat capacity of the calorimeter is the quantity
of heat required to raise the temperature one degree
![]() (6-3)
(6-3)
But there are two sources of heat, the burning
sample and the burning wire.
Thus, the heat capacity is
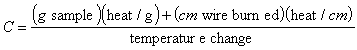 (6-4)
(6-4)
A similar relation is used to determine the heat
of combustion.
![]() (6-5)
(6-5)
Again the heat sources are sample and wire; so,
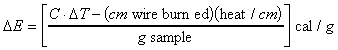
Since combustion occurs at constant volume rather
than constant pressure, the heat of combustion is calculated as DE
rather than DH.
But, DH can be calculated
by use of Equations 6-2.
This experimental value may be compared with
accepted values given in various handbooks. The error in this experiment
is normally small. Using the heat of combustion, the heat of formation
of the sample may be found.
![]() (6-6)
(6-6)
The heats of formation of several related compounds
may be determined. The changes in heat of formation can then be correlated
to structural changes.
The Bomb Calorimeter
Brief Operating Instructions
For more details see Oxygen Bomb Calorimetry
and Combustion Methods, Parr Manual 130. Manual.
- Cut a 10 cm length of fuse wire. Tie it securely to bomb electrodes. (See Fig. 6-2)
- Weigh on an analytical balance one benzoic acid pellet. Benzoic acid produces 6318 cal/g and is used to "standardize" the instrument.
- Place the metal combustion capsule in the electrode holder, and place the pellet in the capsule. Adjust the fuse wires so that they touch the pellet. Avoid short circuits by not letting the wire touch the sample pan.
- Place the sample holder in the bomb. Avoid rapid movement to make sure the wire stays in contact with pellet.
- Screw the top of the bomb as tightly as possible by hand.
- Remove the screw at the top of the bomb and attach the oxygen hose by hand.
- Make sure the small valve on the pressure regulator is off (clockwise). Then open the main valve on the tank. The small gauge indicates the tank pressure.
- Slowly open the small valve (counterclockwise) until the large gauge reads 25-30 atmospheres pressure. Then close the valve. The needle will slowly drop back toward zero.
- Release the pressure in the line by depressing the lever where the line is attached. Disconnect the hose from the bomb.
- Partially screw on the cap. Push it down to release the oxygen and air. Then refill the bomb with oxygen (steps 6-9).
- Place the bomb in the steel bucket. Attach the wire to connect the fuse wire to the transformer. Make sure the wire connector does not touch the bomb anywhere except the proper post.
- Accurately measure 2.000 liters distilled water in a volumetric flask. Pour into the bucket. Watch for bubbles, which indicate leaks.
- Place the top on the apparatus. connect the stirrer wheel to the motor with the belt. Turn on the motor.
- Carefully place the rubber washer on the thermometer (between 22-23oC) Carefully place the thermometer on the support rod. (This is a very expensive thermometer!!!!)
- Record the temperature for several minutes to make sure the temperature is constant.
- Attach the transformer to the calorimeter with the wires provided.
- Fire the bomb by depressing the black button on the transformer. Watch the red light. The red light should go on and then off. If it stays on there is a short. If it does no go on the circuit is open. In either case the apparatus should be dismantled to find the cause.
- Record the temperature rise for several minutes or until the maximum, is passed.
- Dismantle the apparatus. Release the pressure inside the bomb. If there are carbon deposits inside the bomb the results are invalid.
- Measure the length of fuse wire remaining.
- Clean and dry the apparatus.
- Repeat with a sample of unknown heat of combustion.
- Weigh approximately one gram of sample. Use no more than 1.1 gram.
- Use the pellet press to make a pellet. Then accurately weigh the pellet on the analytical balance. (Figure 6-3)
- For volatile samples, see p. 26-27 of manual.
- Repeat steps 1-21 with the sample.
Figure 6-1 Cross section of Parr plain calorimeter.
A. Thermometer
B. Inner Jacket (bucket)
C. Bomb
D. Stirrer
E. Stirring Motor
F. Wire to firing mechanism
Figure 6-2 Single valve bomb with enlarged view
of sample holder and fuse wire.
PELLET MAKING WITH A PARR PRESS
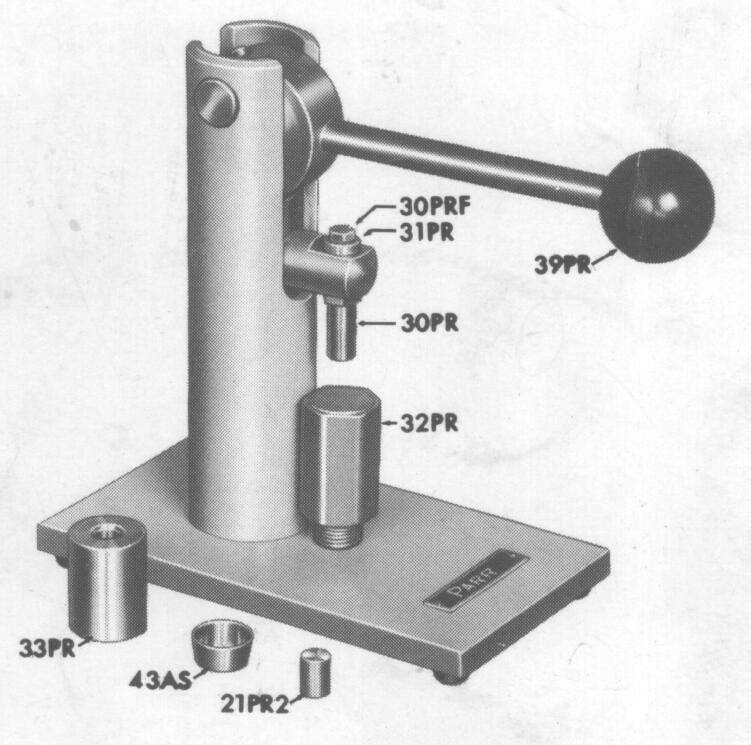
Set the
die (33PR) over the receiving cup (43AS) with these parts resting on the
base of the press or on any flat surface with a square edge. Drop the plug
(21PR) into the die, then fill with the material to be compressed.
Transfer
the die, cup and plug onto the anvil (32PR), holding one finger against
the bottom of the cup to keep it and the plug in place. Compress the charge
by pushing the lever down. Raise or lower the die by screwing the anvil
up or down until firm pressure is required to push the lever through its
full stroke.
Raise
the lever, slide the die from the anvil and remove the cup and plug. Pick
up the plug and drop it into the top of the die above the pellet: then
return the cup and die to their original position on the anvil.
Bring
the lever down gently to eject the pellet into the cup. Be careful not
to move the lever through a full stroke as this might crush the pellet.
Raise
the lever and slide the parts from the anvil. The finished pellet now lies
in the cup. Remove the pellet with tweezers or forceps and repeat the cycle
if additional pellets are required.
Problem 6.1 Caloric Value of Foods
Problem: Which type of food produces the most heat per gram - Protein, fat or carbohydrate?
Directions: Determine the heat of combustion for a protein (albumin, wheat, gluten, etc.), a fat (tristearin, triolein, etc.), and a carbohydrate (sucrose, glucose, starch, etc.). Determine which type of food is of the highest energy.
Experiment: Exp. 6.1 Bomb Calorimeter
Sample Data:
m = 0.969 g
l = 6.2 cm
Dt = 2.53 °C
m = 0.98 g
l = 4.7 cm
Dt = 1.62 °C
Calculations:
Determining the calorimeter constant:
![]()
![]()
![]()
Determining the heat of combustion of a food product:
![]()
![]()
From the Skittles package, we calculate
![]()
![]()